Aliveoss-
Advancing Bone Health at the Molecular Level.
Revolutionizing Bone Healing and Regeneration.
Experience in Osteobiologic Products
Devices used in various clinical applications

About Octum
At Octum Pharmaceuticals, we are dedicated to advancing healthcare by providing innovative and reliable bone graft solutions. With a commitment to excellence, we are providing high-quality bone graft materials designed to support regenerative medicine, orthopedic surgery, and dental procedures.
Our state-of-the-art facilities, cutting-edge technology, and stringent quality standards ensure that every product meets the highest industry benchmarks for safety, efficacy, and performance. Guided by a team of experts in biotechnology, material science, and clinical applications, we strive to deliver solutions that enhance patient outcomes and empower healthcare professionals worldwide.
Our Products
Aliveoss Bone Graft Putty
Place an order or request for more information
Applied NanoBiology Explained:
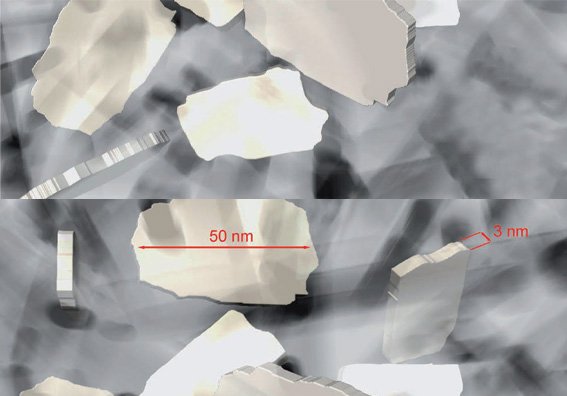
Nano Beta Tricalcium Phosphate
- Bioidentical β-TCP
- Autologous proteins adsorb rapidly to surface
Amorphous Silica Gel Matrix (ASG)
- Rapidly transforms into an osteogenic matrix
- Releases SiO2 triggering angiogenesis, the basis for bone formation
- Highly nanoporous with large internal surface area
- Extremely hydrophilic
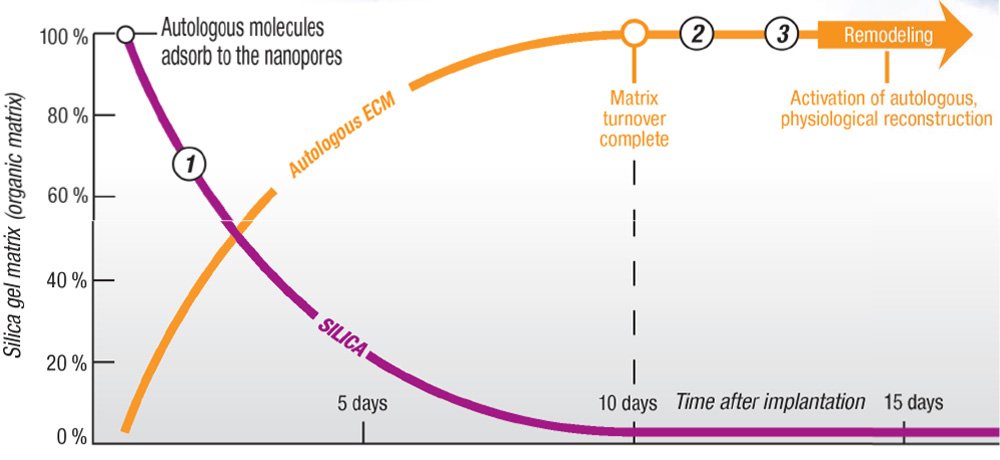
- Post-implantation, release of SiO2 triggers angiogenesis, enhances osteoblastic differentiation, and stimulates bone
formation. Silica gel is transformed into an osteogenic scaffold. Once the organic matrix is in place, osteogenesis and
remodeling proceed.
Harnessing the Power of NanoBiology
Testi monial
Frequently Ask Question
Are your bone graft materials biocompatible?
Do you provide customized bone graft solutions?
How do you ensure the quality of your products?
How are the graft materials stored and shipped?
Our bone grafts are stored and shipped under controlled conditions to maintain their integrity and sterility. Specific storage instructions are provided with each product.
When do you use the large Aliveoss® particles and when the small?
Can Aliveoss® also be used without membrane?
Can Aliveoss® be re-sterilized?
Do you offer training or support for healthcare professionals?
Yes, we provide training sessions, workshops, and technical support to ensure healthcare providers are well-equipped to use our products effectively.
Are your grafts safe for patients with allergies or sensitivities?
Do you ship internationally?
Yes, we serve clients globally, subject to compliance with local regulations in each region.
How do bone grafts integrate with the patient’s natural bone?
How can I become a distributor of your products?
Should Aliveoss® be mixed with antibiotics?
Is Aliveoss® safe (transmission of diseases)?
Aliveoss® is synthetic bone graft material. The strictly controlled manufacturing process ensures high quality and safety standards.